3D cell culturing by magnetic levitation
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|

3D cell culturing by Magnetic Levitation Method (MLM) is the culturing of 3D cell tissue by inducing cells treated with magnetic nanoparticle assemblies in spatially varying magnetic fields. It uses neodymium magnetic drivers and promotes cell-to-cell interactions by levitating the cells up to the air/liquid interface of a standard petri dish. The magnetic nanoparticle assemblies consist of magnetic iron oxide nanoparticles, gold nanoparticles, and the polymer polylysine. 3D cell culturing is scalable, with the capability of culturing 500 cells up to millions of cells, or from a single dish to high-throughput low-volume systems.[1][2][3] Once magnetized cultures are generated, they are used as the building block material, or the "ink" for the magnetic 3D bioprinting process.
Overview
[edit]Standard monolayer cell culture on tissue culture plastic does not replicate the complex 3D architecture of in vivo tissue, but has the potential to modify the morphological properties of cells. Despite being subjected to extensive practical testing and quality regulation, tissue culture plastics may still compromise basic life science experiments, and possibly cause misleading drug-screening results on efficacy and toxicity. This method of cell culturing is also observed to produce cells that may lack the characteristics needed for developing tissue regeneration therapies.[1][2][4][5][6][7][8][9]
One such 3D cell culturing system uses biocompatible polymer-based[1] reagents to deliver magnetic nanoparticles to individual cells, so that an applied magnetic driver can levitate cells off the bottom of the cell culture dish and rapidly bring cells together near the air-liquid interface. This initiates cell-cell interactions in the absence of any artificial surface or matrix. Magnetic fields are designed to form 3D multicellular structures, including the expression of extracellular matrix proteins. The matrix, protein expression, and response to exogenous agents of resulting tissue show great similarity to in vivo results.[1]
History
[edit]3D cell culturing by MLM was developed from collaboration between scientists at Rice University and University of Texas MD Anderson Cancer Center in 2008.[1] Since then, 3D cell culturing technology has been licensed and commercialized by Nano3D Biosciences.[10]
The magnetic levitation process
[edit]
The figure on the right shows 3D cell culturing through magnetic levitation using one possible system. Letters on the figure refer to the following:
(A) A magnetic iron oxide nanoparticle assembly known as the "nanoshuttle" is added and dispersed over cells, and the mixture is incubated.
(B) After incubation with the nanoshuttle, the cells are detached and transferred to a petri dish.
(C) A magnetic drive is then placed on top of a petri dish top.
(D) The magnetic field causes cells to rise to the air–medium interface.
(E) Human umbilical vein endothelial cells (HUVEC) levitated for 60 minutes (left two images in E) and 4 hours (right two images in E) (scale bar, 50 μm).
The onset of cell-cell interaction takes place as soon as cells levitate, and 3D structures start to form. At 1 hour, the cells are still relatively dispersed, but they already show some signs of stretching. Formation of 3D structures is visible after 4 hours of levitation (arrows in E).[1][2]
Protein expression
[edit]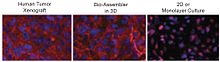
Patterns of protein expression in levitated cultures bear resemblance to the patterns observed in-vivo. For instance, as shown in the figure on the right, N-cadherin expression in levitated human glioblastoma (GBM) cells was nearly identical to the expression seen in human tumor xenografts grown in immunodeficient mice (comparing the left and middle images), while standard 2D culture showed much weaker expression that did not match xenograft distribution (comparing the left and right images).[1] The transmembrane protein N-cadherin is often used as an indicator of in vivo-like tissue assembly in 3D culturing.[1]
Referring to the figure, in the mouse and levitated culture (left and middle image), N-cadherin is clearly concentrated in the membrane, and also present in cytoplasm and cell junctions, whereas the 2D system (right image) shows N-cadherin in the cytoplasm and nucleus, but notably absent from the membrane.[1]
Applications
[edit]
Co-culturing, magnetic manipulation, and invasion assays
[edit]One of the challenges in generating in vivo-like cultures or tissue in vitro is the difficulty in co-culturing different cell types. Co-culturing of different cell types can be achieved at the onset of levitation, either by mixing different cell types before levitation, or by magnetically guiding 3D cultures in an invasion assay format.[1]
Co-culturing in a realistic tissue architecture is important for accurately modeling in vivo conditions, such as for increasing the accuracy of cellular assays, as shown in the figure on the right.[1] In the figure, the human GBM cells and normal human astrocytes (NHA) are cultured separately and then magnetically guided together (left, time 0). Invasion of GBM into NHA in 3D culture provides an assay for basic cancer biology and drug screening (right, 12h to 252h).[1][2]
Vascular simulation with stem cells
[edit]By facilitating the assembly of different populations of cells using the MLM, consistent generation of organoids termed adipospheres capable of simulating the complex intercellular interactions of endogenous white adipose tissue (WAT) can be achieved.[11]
Co-culturing 3T3-L1 pre-adipocytes in 3D with murine endothelial bEND.3 cells create a vascular-like network assembly with concomitant lipogenesis in perivascular cells (refer to the attached figure).[11]
In addition to cell lines, organogenesis of WAT can be simulated from primary cells.[11]
Adipocyte-depleted stromal vascular fraction (SVF) containing adipose stromal cells (ASC), endothelial cells, and infiltrating leukocytes derived from mouse WAT were cultured in 3D. This revealed organoids striking in hierarchical organization with distinct capsules and internal large vessel-like structures lined with endothelial cells, as well as perivascular localization of ASC.[11]
Upon adipogenesis induction of either 3T3-L1 adipospheres or adipospheres derived from SVF, the cells efficiently formed large lipid droplets typical of white adipocytes in vivo, whereas only smaller lipid droplet formation is achievable in 2D. This indicates intercellular signaling that better recapitulates WAT organogenesis.[11]
This MLM for 3D co-culturing creates adipospheres appropriate for WAT modeling ex vivo and provides a new platform for functional screens to identify molecules bioactive toward individual adipose cell populations. It can also be adopted for WAT transplantation applications and aid other approaches to WAT-based cell therapy.[11]

Organized co-culturing to create in vivo-like tissue
[edit]The use of additional manipulation tools may be needed to organize 3D co-cultures into a configuration similar enough to native tissue architecture.
Endothelial cells (PEC), smooth muscle cells (SMC), fibroblasts (PF), and epithelial cells (EpiC) cultured through magnetic levitation can be sequentially layered in a drag-and-drop manner to create bronchioles that maintain phenotype and induce extracellular matrix formation, as shown in the images on the right.[12]
Cell types cultured
[edit]Listed below are the cell types (primary and cell lines) that have been successfully cultured by the magnetic levitation method.
| Cells | Cell line | Organism | Organ tissue | Image |
|---|---|---|---|---|
| Murine endothelial[11] | Cell line | Mouse | Vessel | [11] |
| Murine adipocyte[11] | Cell line | Mouse | Adipose |  |
| Rattus norvegicus hepatoma[citation needed] | Cell line | Rat | Liver |  |
| Human pulmonary fibroblasts (HPF)[12] | Primary | Human | Lung |  |
| Pulmonary endothelial (HPMEC)[12] | Primary | Human | Lung | |
| Small airway epithelial (HSAEpiC)[12] | Primary | Human | Lung |  |
| Bronchial epithelial[12] | Primary | Human | Lung | |
| Human alveolar adenocarcinoma[12] | A549 | Human | Lung |   |
| Type II alveolar[12] | Primary | Human | Lung | |
| Human tracheal smooth muscle cells (HTSMCs)[12] | Primary | Human | Lung |  |
| Human mesenchymal stem cells (HMSCs)[citation needed] | Primary | Human | Bone marrow |  |
| Human bone marrow endothelial cells (HBMECs)[citation needed] | Primary | Human | Bone marrow | |
| Dental pulp stem cells (DPSCs)[citation needed] | Primary | Human |  | |
| Human umbilical vein endothelial cells (HUVECs)[citation needed] | Primary | Human |  | |
| Murine chondrocytes[citation needed] | Primary | Mouse | Bone |  |
| Murine adipose tissue[11] | Primary | Mouse |  | |
| Heart valve endothelial[citation needed] | Primary | Porcine | ||
| Pre-adipocytes fibroblasts[11] | 3T3 | Mouse | ||
| Neural stem cells[citation needed] | C17.2 | Mouse | Brain |  |
| Human embryonic kidney cells[citation needed] | HEK293 | Human | Kidney |  |
| [[Melanoma[citation needed]]] | B16 | Mouse | Skin | |
| Astrocytes[1] | NHA | Human | Brain |  |
| Glioblastomas[1] | LN229 | Human | Brain |  |
| T-cells and antigen presenting cells[citation needed] | Human | |||
| Mammary epithelial[citation needed] | MCF10A | Human | Breast |  |
| Breast cancer[citation needed] | MDA231 | Human | Breast | |
| Osteosarcoma[citation needed] | MG63 | Human | Bone |
References
[edit]- ^ a b c d e f g h i j k l m n o p q r Souza, G. R. et al. Three-dimensional Tissue Culture Based on Magnetic Cell Levitation. Nature Nanotechnol.5, 291-296, doi:10.1038/nnano.2010.23 (2010).
- ^ a b c d Molina, J., Hayashi, Y., Stephens, C. & Georgescu, M.-M. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia12, 453-463 (2010).
- ^ "Bio-Assembling in 3-D with Magnetic Levitation - Technology Review." Technology Review. N.p., n.d. Web. 20 Aug. 2012. <http://www.technologyreview.com/view/426363/bio-assembling-in-3-d-with-magnetic-levitation/>.
- ^ Pampaloni, F., Reynaud, E. G. & Stelzer, E. H. K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol.8, 839-845 (2007).
- ^ Cukierman, E., Pankov, R., Stevens, D. R. & Yamada, K. M. Taking Cell-Matrix Adhesions to the Third Dimension. Science294, 1708-1712 (2001).
- ^ Abbott, A. Biology's new dimension. Nature424, 870-872 (2003).
- ^ Prestwich, G. D. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: A pragmatic approach. J. Cell. Biochem.101, 1370-1383, doi:10.1002/jcb.21386 (2007).
- ^ Boudreau, N. & Weaver, V. Forcing the Third Dimension. Cell125, 429-431 (2006).
- ^ Griffith, L. G. & Swartz, M. A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol.7, 211-224 (2006).
- ^ "N3D Biosciences, Inc. » ABOUT US." N3D Biosciences, Inc. » ABOUT US. N.p., n.d. Web. 20 Aug. 2012. <http://www.n3dbio.com/about/ Archived 2013-12-14 at the Wayback Machine>.
- ^ a b c d e f g h i j k l m Daquinag, A. C., Souza, G. R., Kolonin, M. G. Adipose Tissue Engineering in Three-Dimensional Levitation Tissue Culture System Based on Magnetic Nanoparticles. Tissue Eng. Part C. -Not available-, ahead of print. doi:10.1089/ten.tec.2012.0198 (2012).
- ^ a b c d e f g h i j k l Tseng, H., Gage, J. A., Raphael, R., Moore, R. H., Killian, T. C., Grande-Allen, K. J., Souza, G. R. Assembly of a three-dimensional multitype bronchiole co-culture model using magnetic levitation. Tissue Eng. Part C. -Not available-, ahead of print. doi:10.1089/ten.TEC.2012.0157 (2013)
